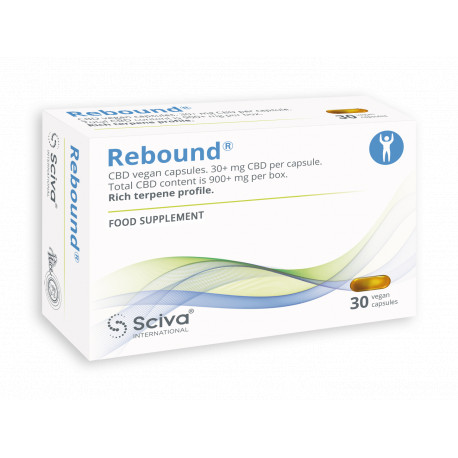
Rebound®, CBD Vegan capsules. Rich terpene profile
CBD Capsules contain full spectrum CBD oil 6% in organic sesame seed oil, 40+ terpenes and flavonoids.
CBD/CBDA: 30 mg/capsule
Total content CBD/CBDA is 900+mg per box.
Terpenes: 22,67 mg/g
Packaging: 30 cps/box
10 cps/blister
Suitable for all diets and religions, 100% plant based.
More info
Rebound®
Food supplement
Manufactured by Pharmaceutical EU GMP and FDA certified factory.
Certification is available on request.
Under Novel Food registration process by EFSA*.
The product is registered by the Australian Government, Department of Health, Therapeutic Goods Administration (TGA).
Composition:
One capsule contains 525 mg high GMP quality CBD enriched Organic Sesame Seed Oil. 30 mg+ CBD per capsule.
|
Active ingredient |
cps. |
RDD* |
|
CBD enriched sesame oil (CBD >_6%) |
525 mg |
NA |
*RDD-Recomended Daily Dose
Additives:
Silicon Dioxide (Processing Aid), vegan capsule (hydroxy- propyl-methyl cellulose).
For oral use only. The product is manufactured under strict adherence to the requirements of good manufacturing practice (GMP), documented by the internationally valid GMP certificate and is verified by regular inspections by the State Institute for Drug Control. FDA certified manufacturer.
Recommended dosage:
1 capsule/day (drink with water 15 min before food).
This product is not suitable for children under 3 years old, pregnant and breastfeeding women.
Don’t exceed the recommended daily dose.
Product is not a substitute for a normal diet. Take care to have a balanced diet and healthy life style. Natural product suitable for vegans.
Storage:
Keep out of reach of children. Keep in a cold, dark and dry place at the temperature up to 30 °C in the original packaging to protect the product from light and moisture.
Shelf Life:
24 months.
* In November 2015, the European Parliament and the Council of the European Union adopted a new regulation on Novel Food, Regulation (EU) 2015/2283, with the intent of making the Novel Food authorization process more efficient while ensuring high standards of food safety or consumers. This new Regulation came into force on 1 January 2018. If lawfully marketed in the EU after 15 May 1997, existing Food Supplement products with Cannabidiol and/or Cannabis Sativa Extract can remain on the market as long as an application for Novel Food authorization is submitted to the European Commission before 1 January 2019.
Testimonials (2)
Results are amazing
Wonderful products and friendly, knowledgeable people. I have loved every product we have tried and the results are amazing. We highly recommend them.
Lifetime customer
1st dose of Rebound and pain-free from neuropathy. I hope this continues to work. All natural and no hidden ingredients. Thank you so much! Lifetime customer.